Abstract
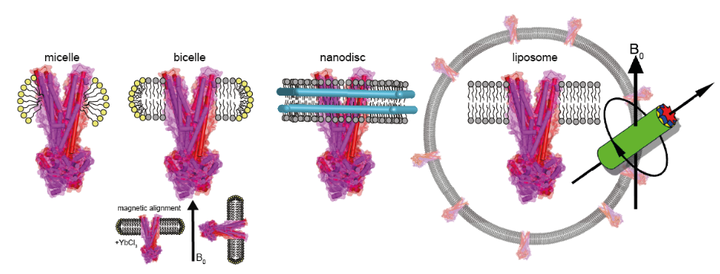
ATP binding cassette (ABC) transporters form a superfamily of integral membrane proteins involved in translocation of substrates across the membrane driven by ATP hydrolysis. Despite available crystal structures and extensive biochemical data, many open questions regarding their transport mechanisms remain. Therefore, there is a need to explore spectroscopic techniques such as solid state NMR in order to bridge the gap between structural and mechanistic data. In this study, we investigate the feasibility of using Escherichia coli MsbA as a model ABC transporter for solid state NMR studies. We show that optimised solubilisation and reconstitution procedures enable preparing stable and homogenous protein samples. Depending on the duration of solubilisation, MsbA can be obtained in either an apo- or in a native lipid A bound form. Building onto these optimisations, the first promising MAS-NMR spectra with narrow lines have been recorded. However, further sensitivity improvements are required so that complex NMR experiments can be recorded within a reasonable amount of time. We therefore demonstrate the usability of paramagnetic doping for rapid data acquisition and explore dynamic nuclear polarisation as a method for general signal enhancement. Our results demonstrate that solid state NMR provides an opportunity to address important biological questions related to complex mechanisms of ABC transporters.